About ozone
What is ozone?
Ozone (O3), which is also called “active oxygen”, is a gas with a molecule containing three oxygen atoms instead of the two in the oxygen that we breathe. That is, ozone is an oxygen molecule (O2) with an extra oxygen atom.
Since ozone is a highly unstable gas, it quickly reverts to its original, stable oxygen molecule form with two atoms (O2), while the third oxygen atom just released immediately reacts with the material that we have exposed to the impact of the ozone. As a result, ozone is an excellent disinfectant, one of the strongest available for daily use.<br /> <br /> It oxidizes bacteria, aspergillus, other fungi, mites and other pathogens (e.g. most viruses and other compounds). It is an excellent solution for eliminating unpleasant odours.<br /> <br /> The effectiveness of ozone is basically due to its rapid oxidation. When encountering various pollutants floating in the air or stuck to surfaces, it reacts with them and breaks up their structure through oxidation. Because of the molecule’s third oxygen atom, ozone is highly reactive (it is an active chemical) – it easily reacts with pollutants, such as odour and pathogen particles, and breaks them into less complex and usually less harmful molecules. The remaining ozone transforms into oxygen, so it is a completely eco-friendly method of disinfection. It is also more effective to clean rooms with ozone than with traditional chemicals.<br /> <br /> Ozone is also created through natural processes. It appears most frequently because of lightning during rainstorms. Actually, the “fresh, clean, springy” smell that we notice after a storm is often linked to ozone. We hear the most about ozone, however, in relation to the ozone layer that surrounds our planet and its atmosphere and protects us from harmful ultraviolet rays. The ozone layer is produced by UV rays from the sun.
How do we produce ozone?
Ozone is naturally created either by ultraviolet radiation (which is a photochemical reaction) or lightning (which is a bioelectric reaction).<br /> <br /> Since ozone becomes oxygen within a relatively short time, it cannot be stored and delivered in a container or bottle. Instead, we produce ozone on the spot with the ozone generator. Two methods that follow the principles of natural ozone production are used to create ozone artificially: ultraviolet light or what is known as corona discharge. The basis for both methods is decomposing oxygen molecules (O2) into two oxygen atoms by adding extra energy and breaking the molecular bond between the two atoms, which then bond to other oxygen molecules (O2), thus creating ozone (O3).<br /> <br /> In the course of corona discharge, we create numerous “miniature lightning bolts” which produce ozone by high voltage on a metal or graphite grid. Ultraviolet (UV) light – with a wavelength of 254nm – also creates ozone out of oxygen molecules. Ozone produced artificially is as effective as ozone formed naturally, provided it is used in the proper concentration.<br /> <br /> In practice, ozone production typically involves the corona discharge method, as it is the most cost-effective: first, the expense of producing ozone is lower, and, second, the ozone generator itself lasts much longer. The gas fed into it may be atmospheric air or pure oxygen. A higher level of ozone concentration can be achieved if pure oxygen is used as a source gas, but that is usually done in special environments.
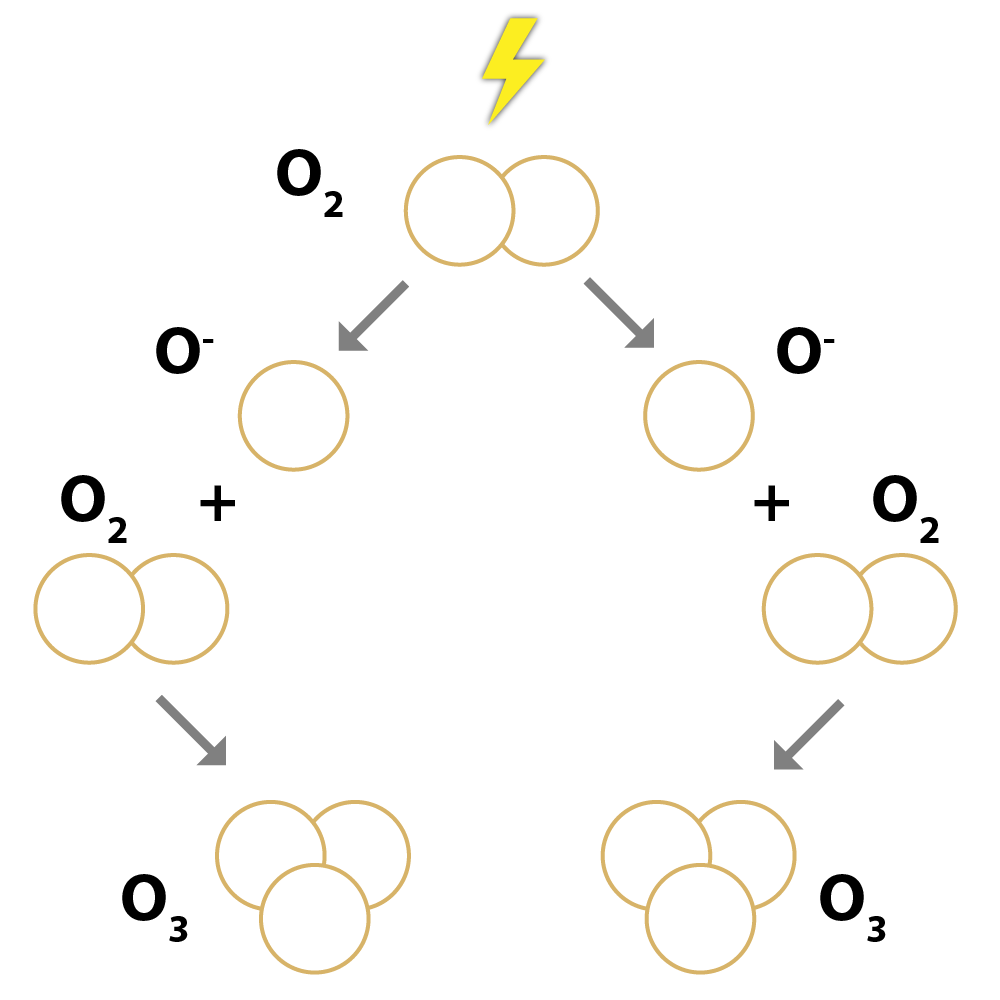
Corona discharge
OzoneMaster ozone generators use the corona discharge method, simulating the natural processes that occur in the production of ozone when lightning strikes. The units that produce ozone are discharge electrodes fed by high voltage (approx. 3kV or more). The electric discharge thus created tears the ozone molecules apart, thus producing two unstable oxygen atoms. These oxygen atoms then combine with the unbroken oxygen molecules so that ozone is created.<br /> <br /> Corona discharge is a simple and economical solution, so ozone generators do not need a temporary storage device that would have to be delivered to the site of use. It is sufficient to put the plug in the socket, and ozone production commences. Highly durable, the electrodes can withstand as many as 3000–6000 hours with a moisture content of approx. 40% – this translates to about 2–4 years of operation at four hours/day on average. Ozone generators have a range of uses, for example, deodorizing and removing mould from air conditioners and disinfecting flats, hotel rooms, gyms, saunas and rooms where animals are kept, as well as upholstery, carpets, bed linen, clothes and other objects. It is extremely effective against bacteria and viruses as well, so it is also indispensable in various health care institutions.
How long does ozone linger in the air?
This process is the outcome of various factors. For one, the ozone reacts oxidatively with organic substances, such as odours or smoke, immediately after it is produced. Moreover, it also reacts with bacteria, viruses and aspergillus, among other pathogens, which continue “to consume” ozone through oxidative reactions. Since ozone reacts with them and many other compounds, the ozone concentration drops fast. In addition, ozone breaks down thermally as well; that is, ozone decomposes faster in high temperature than in low temperature.<br /> <br /> When the majority of the compounds have already oxidized, but the production of ozone has not yet come to a halt (or too much ozone remains in the air), the ozone concentration no longer drops as fast. The remaining ozone turns into oxygen in line with the half-life of ozone.<br /> <br /> Ozone thus does not remain in the air for long despite its powerful abilities. It performs its function and then disappears without a trace, transforming into safe oxygen.
Features of ozone
page-about-ozone-section-5-excerpt
In addition to hydroxyls, ozone is one of the strongest oxidants that occur naturally – it not only protects life on Earth from the harmful effects of the sun’s ultraviolet rays, but it is also highly effective against bacteria, fungi and other pathogens, such as certain viruses and unpleasant odours. Ozone is present in the upper atmosphere, and it is what makes the sky blue. It is formed at an altitude of 25–30km out of a section of the sun’s ultraviolet radiation spectrum – and it exists at that altitude in gaseous form at a concentration of 10–20ppm. At this concentration, ozone absorbs ultraviolet radiation, which is hazardous to life on Earth. Ozone is also present at very low concentrations (0.001–0.003ppm) near the surface of the Earth. It is formed naturally through lightning, but it may also appear near waterfalls or through raging waves on the water.
A COMPARISON OF THE FEATURES OF OZONE AND OXYGEN
HALF-LIFE OF OZONE IN AIR DEPENDING ON TEMPERATURE
HALF-LIFE OF OZONE IN WATER DEPENDING ON TEMPERATURE
*The values above were determined solely on the basis of the thermal decomposition of ozone, so they do not take into account humidity, microbial activity and other effects that occur in the environment.
